Rhodium-mediated 18F-oxyfluorination of diazoketones using a fluorine-18-containing hypervalent iodine reagent†
Abstract
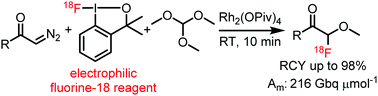
Geminal 18F-oxyfluorination of diazoketones was performed in the presence of rhodium mediators. The reactions were performed using a hypervalent iodine-based [18F]fluoro-benziodoxole reagent. By this methodology various α-[18F]fluoro ethers were obtained in high radiochemical yield (up to 98%) and molar activity (216 GBq μmol−1).


 Please wait while we load your content...
Please wait while we load your content...