Visible light induced redox neutral fragmentation of 1,2-diol derivatives†
Abstract
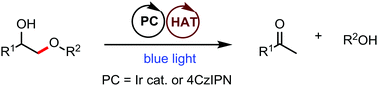
A homogeneous, redox-neutral photo fragmentation of diol derivatives was developed. Under photo/hydrogen atom transfer (HAT) dual catalysis, diol derivatives such as lignin model compounds and diol monoesters undergo selective β C(sp3)–O bond cleavage to afford ketones, phenols and acids effectively.


 Please wait while we load your content...
Please wait while we load your content...