Gold-catalyzed cyclization of 1-(2′-azidoaryl) propynols: synthesis of polysubstituted 4-quinolones†
Abstract
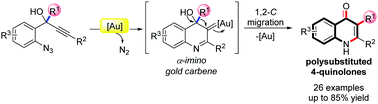
An unprecedented gold-catalyzed procedure for the synthesis of polysubstituted 4-quinolones from 1-(2′-azidoaryl) propynols is described. The reaction undergoes an intramolecular nucleophilic attack of the azide group to the Au-activated triple bonds in a 6-endo-dig manner and subsequent gold-assisted expulsion of N2 to furnish an α-imino gold carbene intermediate, which triggers a 1,2-carbon migration and finally is converted to 2,3-disubstituted 4-quinolone.


 Please wait while we load your content...
Please wait while we load your content...