Direct amide synthesis via Ni-mediated aminocarbonylation of arylboronic acids with CO and nitroarenes†
Abstract
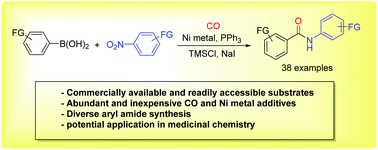
Herein we describe an alternative and unconventional approach of an aminocarbonylation reaction to access aryl amides from readily available and low-cost arylboronic acids and nitroarenes. Nickel metal can serve as both reductant and catalyst in this direct aminocarbonylation. This protocol exhibits a good functional group compatibility and allows a variety of aryl amides to be synthesized, including several drug-like molecules.


 Please wait while we load your content...
Please wait while we load your content...