Selective electroreduction of dinitrogen to ammonia on a molecular iron phthalocyanine/O-MWCNT catalyst under ambient conditions†
Abstract
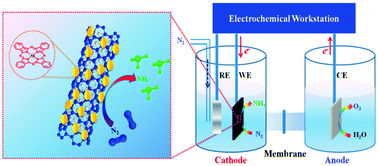
Effective catalysts with sufficient activity and selectivity are essential for the nitrogen reduction reaction (NRR). Many fruitful NRR electrocatalysts have been investigated with regard to NH3 production under ambient conditions in recent years. However, well-defined and modifiable molecular catalysts have rarely been reported for the NRR to date. Here, molecular FePc was grafted on an O-MWCNT surface as a NRR electrocatalyst to improve its recyclability. This catalyst displayed high electrocatalytic ability and selectivity, giving a large NH3 yield of 36 μg h−1 mg−1 cat., a FE of 9.73% and a turnover number (TON) of 12.56 after 2 h of electrocatalytic reaction in an acidic electrolyte, superior to most of the reported materials. DFT calculations indicated that the NRR preferentially proceeded along the alternate pathway, the activation of N2 to produce N2H* is the rate-limiting step with a ΔG value of 1.79 eV. Conclusively, we report FePc/O-MWCNT as a low-cost, high-efficiency NRR catalyst that also offers a valuable reference for molecular electrocatalyst research in electrochemical nitrogen reduction.


 Please wait while we load your content...
Please wait while we load your content...