Isolable diboryl radicals acting as highly efficient reaction intermediates under mild conditions†
Abstract
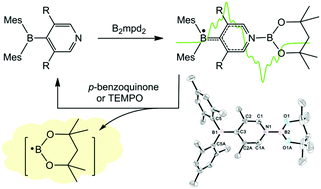
Activation of the B–B bond in diborane B2(OR)4 with dimesitylpyridylboranes 1 and 2 afforded stable diboryl radicals 3 and 4 in moderate yields, respectively, which were studied by single crystal X-ray crystallography, EPR and UV/Vis spectroscopy, in conjunction with theoretical calculations. The diboryl radicals could react with the substrates p-benzoquinone, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and its derivative to form useful boron-containing reagents. Moreover, a much higher yield was achieved in the catalytic reaction of p-benzoquinone and diborane with 1 or 2 in comparison to 4-cyanopyridine as the catalyst, in which the diboryl radicals act as reaction intermediates, highlighting the importance of the stability of the boryl radicals in improving the reaction efficiency.


 Please wait while we load your content...
Please wait while we load your content...