Synthesis of polysubstituted cyclic 1,2-diketones enabled by iterative sulfoxide-mediated arylation†
Abstract
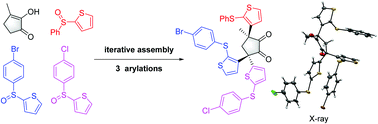
A metal-free α-C–H functionalization of cyclic 1,2-diketones with aryl sulfoxides has been developed. This regioselective arylation involves nucleophilic substitution at the activated sulfoxide with a diosphenol, followed by [3,3]-sigmatropic rearrangement. This protocol can also be applied to the synthesis of polysubstituted cyclic 1,2-diketones with predictable structures by iterative arylations.


 Please wait while we load your content...
Please wait while we load your content...