Recent advances in the synthesis and applications of α-(trifluoromethyl)styrenes in organic synthesis
Abstract
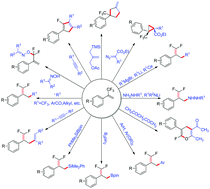
α-Trifluoromethylstyrene derivatives are versatile synthetic intermediates for the preparation of more complex fluorinated compounds. Much attention has been paid to these compounds by the organic chemistry community, because they have been successfully utilized in C–F bond activation in a CF3 group, mainly including anionic SN2′-type substitution, cationic SN1′-type substitution, ipso-substitution and defluorinative functionalization with transition metals or photoredox catalysts. In addition, transition metal-catalyzed cycloaddition reactions of α-trifluoromethylstyrenes have been developed for the construction of cycloalkanes and cycloalkenes containing fluoro or trifluoromethyl groups. In this review, we systematically summarized the synthesis of α-trifluoromethylstyrenes and their applications in organic synthetic chemistry, and their mechanisms were also discussed.


 Please wait while we load your content...
Please wait while we load your content...