Palladium-catalyzed decarboxylative ortho-amidation of O-methyl ketoximes with oxamic acids†
Abstract
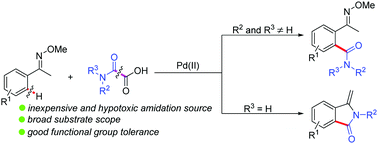
The first palladium-catalyzed ortho-amidation of ketoximes has been developed with readily available, easy to handle and environment-friendly N,N-disubstituted oxamic acids as the amidation sources. When N-monosubstituted oxamic acids are used as the substrates, the formed ortho-amidated ketoximes undergo further intramolecular cyclization to provide 3-methyleneisoindolinones.


 Please wait while we load your content...
Please wait while we load your content...