Cofactor specificity engineering of a long-chain secondary alcohol dehydrogenase from Micrococcus luteus for redox-neutral biotransformation of fatty acids†
Abstract
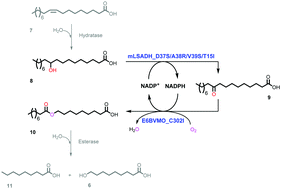
Structure-based engineering of a NAD+-dependent secondary alcohol dehydrogenase from Micrococcus luteus led to a 1800-fold increase in catalytic efficiency for NADP+. Furthermore, the engineered enzymes (e.g., D37S/A38R/V39S/T15I) were successfully coupled to a NADPH-dependent Baeyer–Villiger monooxygenase from Pseudomonas putida KT2440 for redox-neutral biotransformations of C18 fatty acids into C9 chemicals.


 Please wait while we load your content...
Please wait while we load your content...