Water enables an asymmetric cross reaction of α-keto acids with α-keto esters for the synthesis of quaternary isotetronic acids†
Abstract
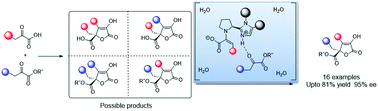
A water promoted asymmetric aldol/lactonization/enolization cascade reaction of α-keto acids and α-keto esters was developed, affording the first general protocol for the construction of chiral quaternary isotetronic acids with excellent enantioselectivity. Theoretical results indicate that intramolecular ionized enamine intermediates stabilized by water generate zwitterionic transition states in a lower activation energy and higher face selectivity, resulting in high activity and chemo- and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...