Transition metal-free access to 3,4-dihydro-1,2-oxaphosphinine-2-oxides from phosphonochloridates and chalcones through tandem Michael addition and nucleophilic substitution†
Abstract
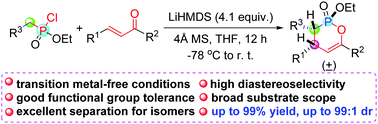
A novel and transition metal-free synthesis of 3,4-dihydro-1,2-oxaphosphinine 2-oxides was developed. LiHMDS-mediated tandem Michael addition and nucleophilic substitution of readily available phosphonochloridates and chalcones afforded a variety of valuable 3,4-dihydro-1,2-oxaphosphinine 2-oxides bearing diverse functionalities in excellent yields and satisfactory to good diastereoselectivity (up to 99% yield and up to 99 : 1 dr).


 Please wait while we load your content...
Please wait while we load your content...