The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide†‡
Abstract
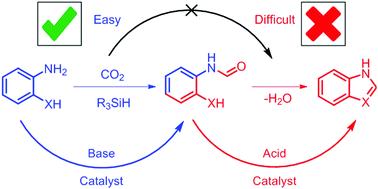
The tandem synthesis of benzimidazole and other azoles can be achived by the N-formylation of ortho-substituted anilines followed by a cyclization reaction. However, CO2-based N-formylations with hydrosilane reducing agents are base catalyzed whereas the cyclization reaction is acid catalyzed. The mismatch in catalytic conditions means that only one of the steps can be catalyzed in a single pot reaction. While the N-formylation reaction is frequently the target of catalyst development, the cyclization reaction requires comparably much harsher reaction conditions. Identification of these difficulties lead us to the development of a one-pot, two-step synthesis of benzimidazole under mild reaction conditions employing acid catalysts.
- This article is part of the themed collections: Celebrating our 2020 Prize and Award winners and Breaking bonds over many timescales: in celebration of Robin Perutz’s 70th birthday


 Please wait while we load your content...
Please wait while we load your content...