Unexpected solvent effect on the binding of positively-charged macrocycles to neutral aromatic hydrocarbons†
Abstract
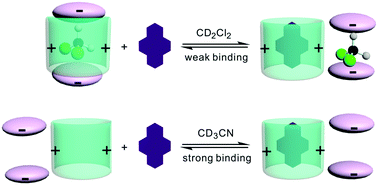
Two positively-charged naphthotubes with imidazolium as the bridges were synthesized and their structures have been characterized by 1H NMR, MS, and X-ray crystallography. These naphthotubes are capable of binding aromatic hydrocarbons. Surprisingly, the binding affinities are much higher in CD3CN than in CD2Cl2. The solvent effect on the ion pairing interactions and competitive binding of CD2Cl2 was invoked to explain this unusual phenomenon.


 Please wait while we load your content...
Please wait while we load your content...