Conformationally stable peptide macrocycles assembled using the Petasis borono-Mannich reaction†
Abstract
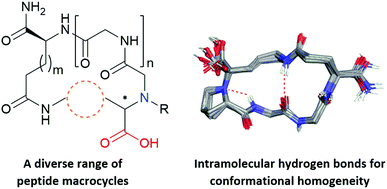
Macrocyclization of linear peptide precursors using the Petasis borono-Mannich reaction affords a diverse range of macrocycles with an endocyclic amine. Analysis of the corresponding macrocyclic structures underscores that the hydrogen bond between an endocyclic amine and the adjacent amide NH is a powerful control element for conformationally homogenous peptide macrocycles.


 Please wait while we load your content...
Please wait while we load your content...