Zn(OAc)2-catalyzed tandem cyclization of isocyanides, α-diazoketones, and anhydrides: a general route to polysubstituted maleimides†
Abstract
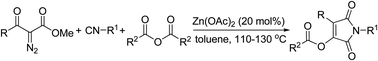
A novel Zn(OAc)2-catalyzed three-component tandem cyclization reaction of isocyanides, α-diazoketones and anhydrides has been developed. The reaction demonstrates the wide scope of substrates and provides a novel and efficient strategy for the synthesis of polysubstituted maleimides from readily available substrates in a single step.


 Please wait while we load your content...
Please wait while we load your content...