CF2H as a hydrogen bond donor group for the fine tuning of peptide bond geometry with difluoromethylated pseudoprolines†
Abstract
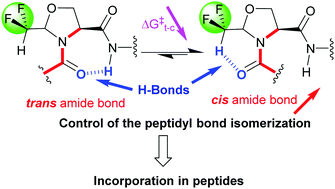
CF2H-Pseudoprolines obtained from difluoroacetaldehyde hemiacetal and serine are stable proline surrogates. The consequence of the incorporation of the CF2H group is an important decrease of the trans to cis amide bond isomerization energy and a remarkable stabilisation of the cis conformer by an hydrogen bond.


 Please wait while we load your content...
Please wait while we load your content...