Tetrahedral iron featuring an appended Lewis acid: distinct pathways for the reduction of hydroxylamine and hydrazine†
Abstract
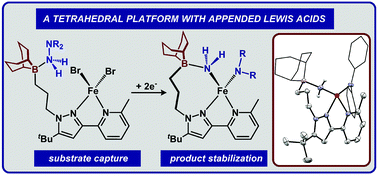
We present the preparation of a nitrogen-based bidentate ligand featuring an appended boron Lewis acid as well as its tetrahedral Fe2+ and Zn2+ complexes. These complexes act as platforms for hydrazine and hydroxylamine capture and reduction chemistry.


 Please wait while we load your content...
Please wait while we load your content...