C–H alkenylation/cyclization and sulfamidation of 2-phenylisatogens using N-oxide as a directing group†
Abstract
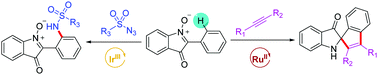
The first example of transition-metal-catalyzed C–H activations of 2-phenylisatogens with alkynes and sulfonyl azides has been developed using N-oxide as the directing group. Ru(II)-Catalyzed C–H alkenylation/cyclization and Ir(III)-catalyzed direct C–H sulfamidation proceeded with good yields and excellent functional group tolerance. Importantly, these two transformations provided straightforward routes for the synthesis of indol-3-one derivatives and sulfamidated 2-phenylisatogens respectively, which might be of considerable bioactivities.


 Please wait while we load your content...
Please wait while we load your content...