The synthesis of densely functionalised α-acyloxy enaminals and enaminones via a novel homogeneous silver(i) catalysed rearrangement†‡
Abstract
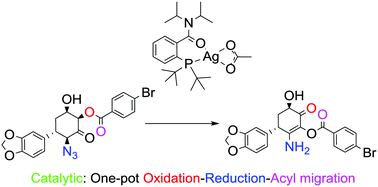
A synthesis of densely functionalised α-acyloxy enaminals and enaminones via a novel homogeneous silver(I) catalyzed rearrangement of 1-acyloxy-3-azido ketones is reported. This silver catalyzed reaction involves an internal redox process comprised of four net transformations: loss of nitrogen, reductive cleavage of the azide, 1,2-acyl migration and oxidation of the acyloxy position to an aldehyde (enaminal) or ketone (enaminone). These mild reaction conditions have been applied to acyclic, cyclic, and chiral substrates yielding the rearranged enaminals or enaminones in up to 91% yield, all of which prove to be stable, isolatable products.


 Please wait while we load your content...
Please wait while we load your content...