5,20-Diheterohexaphyrins: metal-template-free synthesis and aromaticity switching†
Abstract
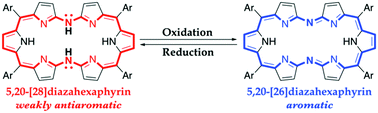
As the first example of expanded heteroporphyrins with heteroatoms at meso-positions, 5,20-dithiahexaphyrins and 5,20-diazahexaphyrins were synthesized via nucleophilic substitution reactions of α,α′-dibromotripyrrin as the key step. While 5,20-dithiahexaphyrins are practically nonaromatic, 5,20-[28]diazahexaphyrins show weakly antiaromatic characters and can be cleanly converted into aromatic 5,20-[26]diazahexaphyrins upon oxidation with PbO2, demonstrating distinct aromaticity switching with maintenance of dumbbell-like conformations.


 Please wait while we load your content...
Please wait while we load your content...