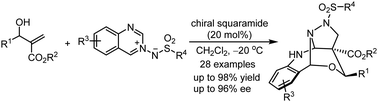
A chiral squaramide-catalyzed asymmetric dearomative tandem annulation reaction through a kinetic resolution of MBH alcohols: highly enantioselective synthesis of three-dimensional heterocyclic compounds†
Abstract
In this communication, a chiral squaramide-catalyzed asymmetric dearomative tandem annulation reaction of unmodified Morita–Baylis–Hillman alcohols with azomethine imines has been achieved through a kinetic resolution of MBH alcohols under mild conditions, giving pharmaceutically interesting functionalized hetero-bicyclo[3.3.1]nonane derivatives in good to excellent yields with excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...