Methane functionalization in water with micellar catalysis†
Abstract
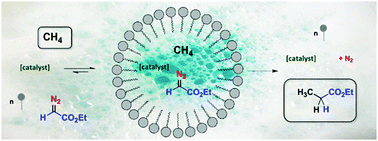
The functionalization of methane in water as the reaction medium (where it is nearly insoluble) at room temperature using micellar catalysis is described. Aggregates are formed from surfactant molecules and act as methane concentrators, also trapping the catalyst (a silver-based complex) and the diazo reagent (ethyl diazoacetate, EDA), providing yields of ethyl propionate up to 14% (referred to as EDA). This is the first example of methane being functionalized in water at room temperature.


 Please wait while we load your content...
Please wait while we load your content...