Ni-Catalyzed deaminative hydroalkylation of internal alkynes†
Abstract
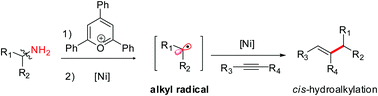
A regioselective cis-hydroalkylation of internal alkynes with readily prepared Katritzky pyridinium salts for the synthesis of tri-substituted alkenes is described. This reaction is the first example of a metal-catalyzed hydroalkylation of an alkyne via C–N bond activation of an amine. The reaction demonstrates broad scope and functional group tolerance, allowing access to desired products with high diversity. Preliminary mechanistic studies indicate that a combination of an SET-initiated radical process and Ni-catalyzed alkylation could engage in the reaction, which makes it possible to bypass the traditional open-shell addition pathway.
- This article is part of the themed collection: SU 120: Celebrating 120 Years of Soochow University


 Please wait while we load your content...
Please wait while we load your content...