A metal-free desulfurizing radical reductive C–C coupling of thiols and alkenes†
Abstract
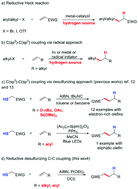
An intermolecular reductive C–C coupling of electrophilic alkyl radicals and alkenes has been developed. Thiols were used as both hydrogen-donating reagents and alkyl radical precursors in the presence of triethyl phosphite and radical initiator. A wide range of alkenes, including styrenes, and aliphatic olefins were well tolerated in this transformation. Mechanistic studies indicated that a phosphite promoted radical desulfurization of thiols to access electrophilic alkyl radicals and a radical chain propagation process may be involved in this transformation.


 Please wait while we load your content...
Please wait while we load your content...