Ceria-reduced graphene oxide nanocomposite as an efficient electrocatalyst towards artificial N2 conversion to NH3 under ambient conditions†
Abstract
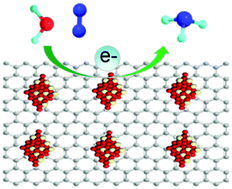
For over a century, NH3 synthesis via the Haber–Bosch process has brought huge energy costs and high CO2 emission. The electrochemical N2 reduction reaction is an environmentally-benign alternative, which can be driven by renewable energy. In this work, CeO2 nanoparticle–reduced graphene oxide nanocomposites (CeO2–rGO) behave as an efficient non-noble-metal N2 reduction reaction electrocatalyst with excellent selectivity. In 0.1 M Na2SO4, CeO2–rGO achieves a high faradaic efficiency of 4.78% and a large NH3 yield of 16.98 μg h−1 mgcat.−1 at −0.7 V vs. reversible hydrogen electrode. The catalytic mechanism was explored using density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...