A counterintuitive stereochemical outcome from a chelation-controlled vinylmetal aldehyde addition leads to the configurational reassignment of phormidolide A†
Abstract
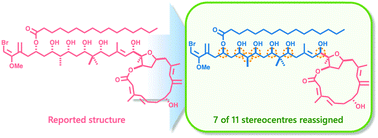
As part of our ongoing studies towards the total synthesis of phormidolide A (1), we explored the chelation-controlled vinylmetal addition of iodide 2 to aldehyde 3 to install the reported 17S configuration. While the stereochemical outcome of this reaction was opposite to that expected, detailed NMR comparisons with the previously reported triacetonide derivative of phormidolide A (18) highlighted that the major adduct was a better match to the natural product. The synthesis of three model acetonides and detailed spectroscopic comparisons to the triacetonide derivative of phormidolide A supports a reassignment of seven of the 11 stereocentres in phormidolide A (1a).


 Please wait while we load your content...
Please wait while we load your content...