The base-catalysed Tamura cycloaddition reaction: calculation, mechanism, isolation of intermediates and asymmetric catalysis†
Abstract
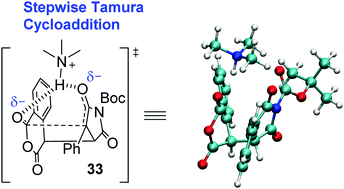
A combined experimental and computational investigation has revealed that the base-catalysed Tamura cycloaddition between homophthalic anhydride and activated alkenes/alkynes – a reaction previously thought of as a Diels–Alder type process – proceeds via a stepwise mechanism involving conjugate addition and ring closure; which allowed the first catalytic asymmetric α-substitution reactions to be demonstrated with up to >99% ee.


 Please wait while we load your content...
Please wait while we load your content...