Evaluation of linker length effects on a BET bromodomain probe†
Abstract
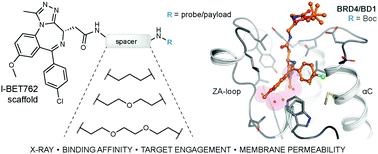
Fueled by the therapeutic potential of the epigenetic machinery, BET bromodomains have seen high interest as drug targets. Herein, we introduce different linkers to a BET bromodomain benzodiazepine ligand (I-BET762) to gauge its implications in the development of hybrid drugs, imaging probes and small molecule drug conjugates. Biophysical studies confirmed minimal disruption to binding of the BRD4 cavity by the synthesized entities, which includes imaging probes. Target engagement was confirmed in a cellular context, but poor membrane diffusion was found despite efficient localization in the nuclei after membrane disruption. Our study highlights challenges and opportunities for the successful design of benzodiazepine-derived drug-delivery systems.


 Please wait while we load your content...
Please wait while we load your content...