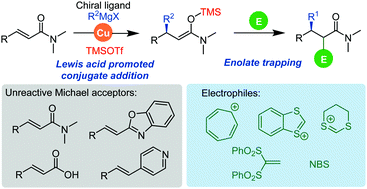
Trapping of chiral enolates generated by Lewis acid promoted conjugate addition of Grignard reagents to unreactive Michael acceptors by various electrophiles†
Abstract
Here we show trapping of chiral enolates with carbenium ions, Michael acceptors, and bromine. Silyl ketene aminals, disilyl acetals, and aza-enolates were obtained via Lewis acid mediated enantioselective conjugate addition of Grignard reagents to unsaturated amides, carboxylic acids and alkenyl heterocycles.


 Please wait while we load your content...
Please wait while we load your content...