Stabilization of a 4.5 V Cr4+/Cr3+ redox reaction in NASICON-type Na3Cr2(PO4)3 by Ti substitution†
Abstract
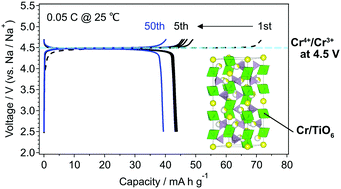
The development of high-voltage cathode materials composed of abundant metals for rechargeable batteries is a crucial task to realize higher energy density in large-scale electrical energy storage systems. Here we report a reversible Cr4+/Cr3+ redox reaction at 4.5 V vs. Na/Na+ in NASICON-type Na2CrTi(PO4)3 (NCTP). An unstable Cr4+/Cr3+ redox in Na3Cr2(PO4)3 is successfully stabilized by the substitution of Ti with Cr. The charge/discharge mechanism of NCTP was studied by powder X-ray diffraction and soft X-ray absorption spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...