Pseudodiborenes: hydride-bridged diboranes(5) as two-electron reductants of chalcogens†
Abstract
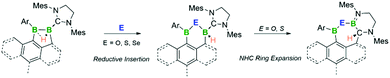
The reactivity of two nucleophilic neutral sp2–sp3 diboranes towards chalcogens is presented herein. Both diboranes(5) serve as two-electron reductants, incorporating oxygen, sulfur and selenium atoms. Treatment with chalcogen sources results in the oxidative insertion of one chalcogen atom into the B–B single bond, while depending on the negative inductive effect of the chalcogen and the boron bound aryl substituent further N-heterocyclic carbene (NHC) ring expansion and hydride migration can occur. These reactions provide access to unprecedented six- or seven-membered heterocycles and help to illuminate the pseudo-multiple bonding character of hydrogen-bridged B–B single bonds.


 Please wait while we load your content...
Please wait while we load your content...