Artificial β-propeller protein-based hydrolases†
Abstract
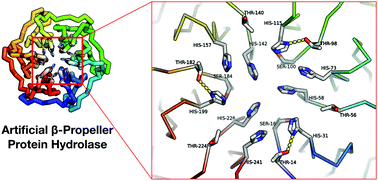
We developed an artificial hydrolase based on the symmetrical Pizza6 β-propeller protein for the metal-free hydrolysis of 4-nitrophenyl acetate and butyrate. Through site-specific mutagenesis and crystallisation studies, the catalytic mechanism was investigated and found to be dependent on a threonine–histidine dyad. The mutant with additional histidine residues generated the highest kcat values, forming a His–His–Thr triad and matched previously reported metalloenzymes. The highly symmetrical β-propeller artificial enzymes and their protein–metal complexes have potential to be utilised in bioinorganic and supramolecular chemistry, as well as being developed further into 2D/3D catalytic materials.
- This article is part of the themed collection: 2019 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...