Hypervalent iodine initiated intramolecular alkene dimerisation: a stereodivergent entry to cyclobutanes†
Abstract
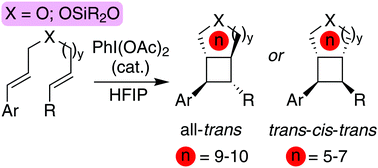
The emergence of new methods for the stereoselective synthesis of strained carbocycles is a challenging but worthwhile endeavour. Cyclobutanes, in particular, have attracted the attention of both medicinal chemists and material scientists for their unique properties. Herein, we present a new method that allows access to highly functionalized cyclobutanes with complementary all-trans and trans–cis–trans relative stereochemistry, that could not be accessed before. This approach consists of an intramolecular dimerisation of non-conjugated dienes using an oxidative single electron transfer (SET) process, and is initiated by catalytic amounts of hypervalent iodine reagents. The potential uses of these cyclobutanes is demonstrated with selective functionalization, including the formation of diols and carboxylic acids.


 Please wait while we load your content...
Please wait while we load your content...