Boosting electrocatalytic nitrogen fixation via energy-efficient anodic oxidation of sodium gluconate†
Abstract
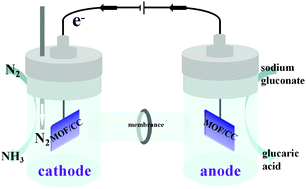
Here, we report an anodic replacement of the water oxidation reaction with more readily oxidizable species to facilitate ambient electrocatalytic nitrogen reduction reaction (NRR). A self-supported catalyst, CuII–MOF on carbon cloth (JUC-1000/CC), acts as a versatile cathode and anode for both NRR and electro-oxidation of sodium gluconate to glucaric acid. Impressively, the two-electrode system requires a potential of only 0.4 V to achieve an NH3 yield rate of 24.7 μg h−1 mgcat−1, an FE of 11.90% and an SA selectivity of 96.96%, and shows strong electrochemical stability. This study reveals that the strategy avoids the sacrifice of the NH3 yield to increase FE, and offers an efficient and simultaneous electrosynthesis of NH3 and SA.


 Please wait while we load your content...
Please wait while we load your content...