Scandium catalysed stereoselective thio-allylation of allenyl-imidates†
Abstract
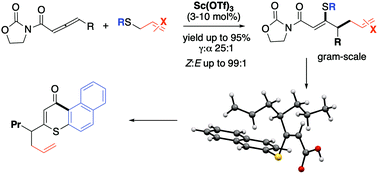
The site-selective thio-allylation of electron-deficient 1,2-dienes is documented under scandium catalysis. The methodology enables the realization of α-β unsaturated, β-thio, γ-allyl carboxylic acid derivatives via a one-pot Lewis acid promoted Michael addition/[3,3]-sigmatropic rearrangement sequence (20 examples) in high yields (up to 95%). Full rationalization of the reaction mechanism and stereochemical outcome is provided via DFT simulations.


 Please wait while we load your content...
Please wait while we load your content...