Targeting the Mycobacterium tuberculosis transpeptidase LdtMt2 with cysteine-reactive inhibitors including ebselen†
Abstract
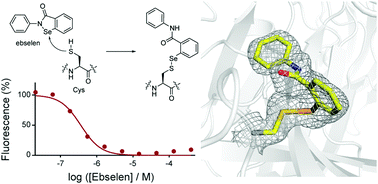
The L,D-transpeptidases (Ldts) are promising antibiotic targets for treating tuberculosis. We report screening of cysteine-reactive inhibitors against LdtMt2 from Mycobacterium tuberculosis. Structural studies on LdtMt2 with potent inhibitor ebselen reveal opening of the benzisoselenazolone ring by a nucleophilic cysteine, forming a complex involving extensive hydrophobic interactions with a substrate-binding loop.


 Please wait while we load your content...
Please wait while we load your content...