Directed ortho-metalation–nucleophilic acyl substitution strategies in deep eutectic solvents: the organolithium base dictates the chemoselectivity†
Abstract
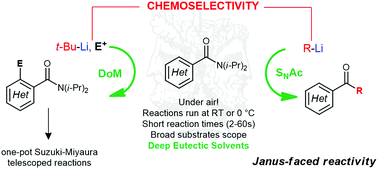
Directed ortho metalation (DoM) or nucleophilic acyl substitution (SNAc) can be efficiently programmed on the same aromatic carboxylic acid amide, in a choline chloride-based eutectic mixture, by simply switching the nature of the organolithium reagent. Telescoped, one-pot ortho-lithiation/Suzuki–Miyaura cross-couplings have also been demonstrated for the first time in Deep Eutectic Solvents.


 Please wait while we load your content...
Please wait while we load your content...