Development of a safely handleable synthetic equivalent of cyanonitrile oxide by 1,3-dipolar cycloaddition of nitroacetonitrile†
Abstract
Dianionic cyano-aci-nitroacetate affords 3-cyanoisoxazol(in)es upon heating with a range of dipolarophiles in the presence of hydrochloric acid. In this reaction, nitroacetonitrile is formed as an intermediate active species, which serves as a synthetic equivalent of cyanonitrile oxide that can participate in a 1,3-dipolar cycloaddition reaction.
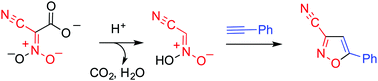


 Please wait while we load your content...
Please wait while we load your content...