Gold(i)-catalyzed cascade cyclization of O-tethered 1,7-enynes bearing a cyclopropane moiety: construction of multi-substituted furans†
Abstract
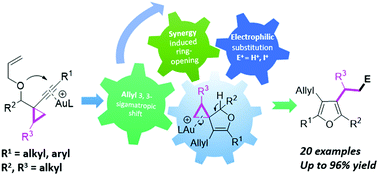
A gold-catalyzed cascade cyclization of O-tethered 1,7-enynes bearing a cyclopropane moiety has been reported in this communication, affording multi-substituted furans in moderate to good yields. The reaction proceeded through an intramolecular O-nucleophilic addition and 3,3-sigmatropic rearrangement followed by ring-opening of the cyclopropane unit along with the further reaction with electrophiles. The C–C bond cleavage of a strained cyclopropane moiety in the presence of a gold catalyst and the aromatization provided the driving force for this cascade cyclization. The practical transformations of the obtained diiodinated furan have also been indicated.


 Please wait while we load your content...
Please wait while we load your content...