Harnessing the reactivity of ortho-formyl-arylketones: base-promoted regiospecific synthesis of functionalized isoquinolines†
Abstract
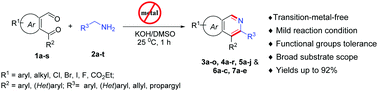
An efficient and base-mediated one-pot regiospecific synthesis of structurally diversified isoquinolines and benzo[h] isoquinolines from easily accessible ortho-formyl-arylketones and aryl/(het)arylmethanamines has been described. Challenging 3-alkynyl/alkenyl isoquinolines and bis-isoquinolines were easily attained through this developed chemistry, which can be further used for various organic transformations. Operational simplicity, high atom-economy, broad substrate scope, functional group tolerance and applicability towards large scale synthesis are the advantageous features of this developed methodology.


 Please wait while we load your content...
Please wait while we load your content...