Intramolecular palladium(ii)/(iv) catalysed C(sp3)–H arylation of tertiary aldehydes using a transient imine directing group†‡
Abstract
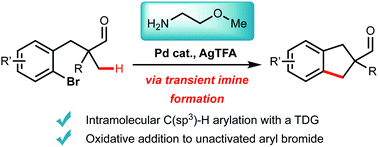
Palladium catalysed β-C(sp3)–H activation of tertiary aldehydes using a transient imine directing group enables intramolecular arylation to form substituted indane-aldehydes. A simple amine bearing a methyl ether (2-methoxyethan-1-amine) is the optimal TDG to promote C–H activation and reaction with an unactivated proximal C–Br bond. Substituent effects are studied in the preparation of various derivatives. Preliminary mechanistic studies identify a reversible C–H activation, product inhibition and suggest that oxidative addition is the turnover limiting step.
- This article is part of the themed collection: Most popular organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...