Traceless synthesis of protein thioesters using enzyme-mediated hydrazinolysis and subsequent self-editing of the cysteinyl prolyl sequence†
Abstract
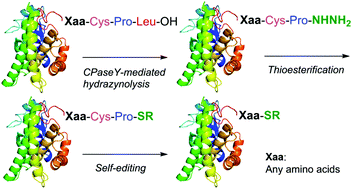
A traceless thioester-producing protocol featuring carboxypeptidase Y-mediated hydrazinolysis of cysteinyl prolyl leucine-tagged peptides has been developed. The hydrazinolysis followed by thioesterification affords cysteinyl prolyl thioesters. Self-editing of the tag and subsequent trans-thioesterification yields peptide thioesters. The developed protocol was successfully applied to the conversion of recombinant proteins to thioesters.


 Please wait while we load your content...
Please wait while we load your content...