Base-catalysed reductive relay hydroboration of allylic alcohols with pinacolborane to form alkylboronic esters†
Abstract
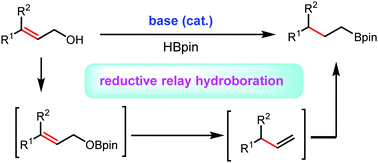
An unprecedented base-catalysed reductive relay hydroboration of allylic alcohols is described. Commercially available nBuLi was found to be a robust transition metal-free initiator for this protocol, affording various boronic esters in high yield and selectivity. Mechanistically, this methodology involves a one-pot three-step successive process (dehydrocoupling/allylic hydride substitution/anti-Markovnikov hydroboration).
- This article is part of the themed collection: 2019 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...