Mechanism and regioselectivity of the anionic oxidative rearrangement of 1,3-diketones towards all-carbon quaternary carboxylates†
Abstract
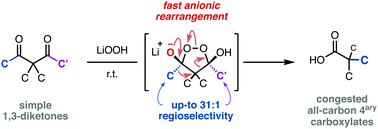
The oxidative rearrangement of 1,3-diketones is an underexplored alternative to enolate chemistry in the synthesis of all-carbon quaternary carboxylates. The mechanistic investigation of this reaction has resulted in a mild base mediated protocol, whose regioselectivity has been studied in challenging acyclic substrates.
- This article is part of the themed collection: 2019 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...