Copper-catalysed, diboron-mediated cis-dideuterated semihydrogenation of alkynes with heavy water†
Abstract
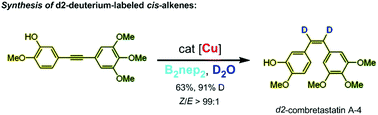
Methods to incorporate deuterium atoms into organic molecules are valuable for the pharmaceutical industry. Here, we found that diboron reagents can efficiently mediate the transfer of two D atoms from heavy water directly onto alkynes through copper-catalysed cis-selective semihydrogenation. Avoiding the use of costly and flammable D2 gas, this safe and practical process can proceed with excellent chemoselectivity and stereoselectivity. Utilizing the present method as the key step, the formal asymmetric total synthesis of d2-deuterium-labeled cis-combretastatin A4 is demonstrated. Mechanistic studies suggest that monoborylation of alkynes is the key step for this semihydrogenation process.


 Please wait while we load your content...
Please wait while we load your content...