TMSOTf mediated ‘5/6-endo-dig’ reductive hydroamination for the stereoselective synthesis of pyrrolidine and piperidine derivatives†
Abstract
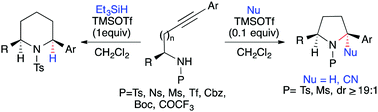
A TMSOTf mediated 5/6-endo-dig reductive hydroamination cascade on internal alkynylamines gave expedient, stereoselective access to pyrrolidine and piperidine derivatives. We also demonstrate that a protecting group on nitrogen has a profound effect on the reactivity as well as diastereoselectivity of the reductive hydroamination cascade.


 Please wait while we load your content...
Please wait while we load your content...