Synthesis of metallophosphaalkenes by reaction of organometallic nucleophiles with a phosphaethynolato-borane†
Abstract
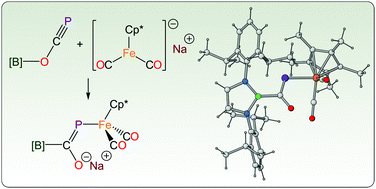
We describe the reaction of a phosphaethynolato-borane [B]OCP ([B] = N,N′-bis(2,6-diisopropylphenyl)-2,3-dihydro-1H-1,3,2-diazaboryl) with the organometallic nucleophile Na[Cp*Fe(CO)2] (Cp* = pentamethylcyclopentadienyl). The electrophilic character of [B]OCP allows for a new route towards the formation metal–phosphorus bonds affording a metallophosphaalkene that can be functionalised at both the oxygen and phosphorus atoms depending on the reagents employed.


 Please wait while we load your content...
Please wait while we load your content...