Triarylalkenes from the site-selective reductive cross-coupling of benzophenones and aldehydes†
Abstract
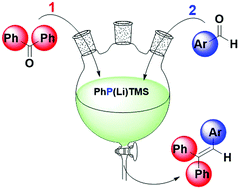
PhP(Li)TMS converts benzophenones to phosphaalkenes which upon activation under oxidizing, basic conditions react with aromatic aldehydes under the formation of triarylalkenes. The one-pot reaction omits transition metals, proceeds at room temperature and precludes the formation of any homo-coupling products. Systematic substrate variations reveal reactivity patterns that are useful for the identification of ketone/aldehyde combinations that can be coupled in yields up to 80%.


 Please wait while we load your content...
Please wait while we load your content...