Recent advances in the catalytic asymmetric alkylation of stabilized phosphorous ylides
Abstract
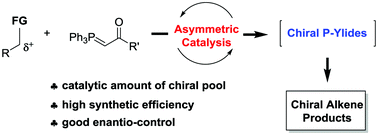
The Wittig reaction is a reliable method for synthesizing alkenes from phosphorous ylides (P-ylides) and carbonyls, and is thus widely applied in medicine and pharmaceutical production. Among them, asymmetric Wittig reactions using chiral P-ylides are believed to be a conventional pathway towards chiral alkenes. Obviously, the key of this transformation is the efficient construction of chiral P-ylides. Over the past decade, the coupling of the in situ generated chiral P-ylides through the catalytic asymmetric alkylation of easily available P-ylides and the subsequent Wittig reaction with carbonyls has already been achieved, allowing the efficient and selective synthesis of versatile chiral alkenes. This review highlights these impressive advances according to the catalysis type, and the general mechanisms and stereochemical inductions are briefly discussed as well. We hope this article will be a useful reference and inspiration for those who are exploring new methods and new applications of chiral P-ylides.
- This article is part of the themed collection: 2019 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...